Antimicrobial Resistance (AMR)

Antimicrobial resistance (AMR) has become one of the world’s greatest threats to public health, with over 1,27 million deaths worldwide today, and an estimated 10 million deaths a year by 20501. In 2023, one in six bacterial infections worldwide was resistant to antibiotics2.
Antimicrobial Resistance
A growing threat to human health…
This not-so-silent pandemic is the result of excessive and inappropriate use of antibiotics over the years, which has led to the emergence of bacterial strains resistant to the effects of antibiotic treatments, reducing their effectiveness.
This increased resistance makes common bacterial infections increasingly difficult to treat and can lead to medical complications and a significant increase in associated morbidity and mortality rates. In addition to the direct impact on human health, AMR exerts considerable economic pressure.
In the US, the overall direct cost of Hospital-acquired infections (HAIs) to hospitals ranges from $28 billion to $45 billion3.
More than 70% of the bacteria that cause HAIs are resistant to at least one of the drugs most commonly used to treat them, and there is strong evidence that the attributable costs, length of stay and mortality are even greater when an infection is caused by a multi-drug-resistant bacterium3.
BBC StoryWorks ad presented by AMR Action Fund with funding support from Shionogi, Pfizer, and MSD.
“AMR is one of the 10 global public health threats facing humanity”
World Health Organization
In 2017, the WHO published a list of 12 antibiotic-resistant “priority pathogens” to guide the R&D of new antibiotics.
In 2023, the WHO further updated this list and published a first global research agenda4 for the world’s scientists to address the most urgent human health priorities to combat Antibiotic Resistance, recognized as an urgent public health and economic challenge for which innovations are desperately needed5-7.
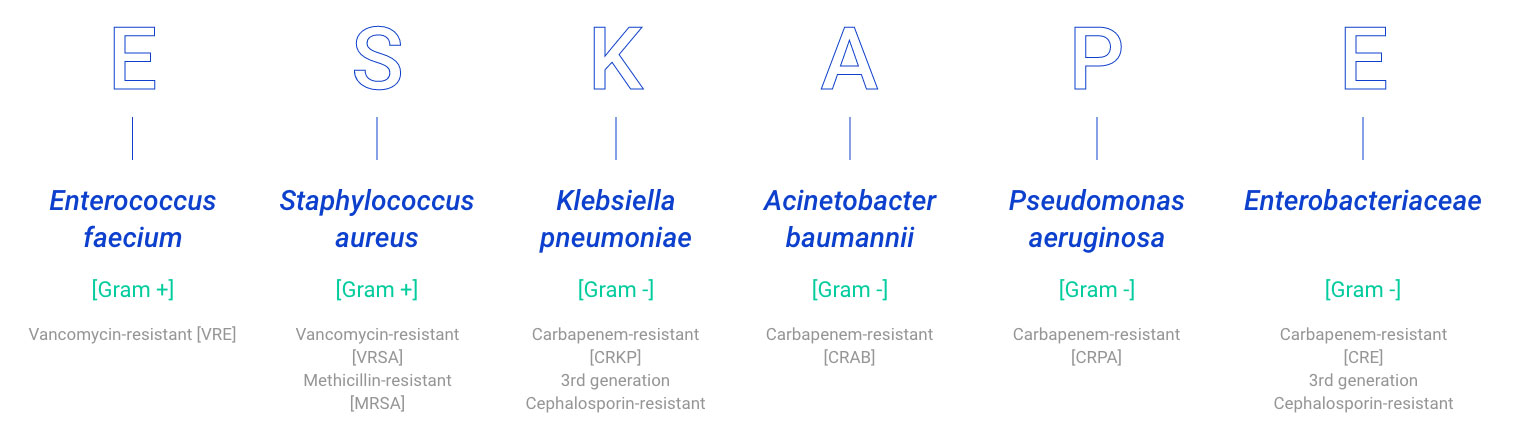
The majority of those pathogens listed as priorities by the WHO are ESKAPE pathogens, a group of opportunistic pathogens consisting of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. These bacteria represent a global threat from a clinical point of view since they exhibit multidrug resistance and virulence. In particular, P. aeruginosa is a ubiquitous Gram-negative bacterium causing several infections, including respiratory infections, that are becoming more difficult to treat because of increasing antibiotic resistance8.
Despite the health emergency, less than 20 new antibacterials have been approved worldwide since 20174-9.
AUROBAC’s strategy is to develop and market the next generation of innovative medicines combined with rapid, precision diagnostics to address high unmet medical needs associated to infections in acute hospital settings, amidst the growing antimicrobial resistance (AMR) epidemic.
Sources
1. Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 399, 629–655 (2022).
2. Global antibiotic resistance surveillance report 2025: WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS): Summary. World Health Organization (2025)
3. Stone, P. W. Economic burden of healthcare-associated infections: an American perspective. Expert Rev. Pharmacoecon. Outcomes Res. 9, 417–422(2009).
4. Global research agenda for antimicrobial resistance in human health. World Health Organization (2023)
5. Prioritization of pathogens to guide discovery, research, and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis.World Health Organization (2017)
6. WHO outlines 40 research priorities on antimicrobial resistance. World Health Organization (2023)
7. Maarten. ECCMID 2023: Updating the Global Bacterial Priority Pathogens List. AMR Insights (2023).
8. Mulani, M. S., Kamble, E. E., Kumkar, S. N., Tawre, M. S. & Pardesi, K. R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 10, 539 (2019).
9. Butler, M. S. et al. Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed. Antimicrob. Agents Chemother. 66, e01991-21 (2022).